by admin | Nov 14, 2018 | Uncategorized

The results of a new clinical trial with ketamine were published in Nature’s Molecular Psychiatry journal. Researchers conducted a double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). 99 subjects, 18-70 years old with TRD (defined as having less than 50% improvement in depression symptoms with conventional therapies) were randomized into one of five arms: a single intravenous dose of ketamine 0.1 mg/kg (n = 18), a single dose of ketamine 0.2 mg/kg (n = 20), a single dose of ketamine 0.5 mg/kg (n = 22), a single dose of ketamine 1.0 mg/kg (n = 20), and a single dose of midazolam 0.045 mg/kg (active placebo) (n = 19).
The authors found that the 0.5 and 1.0 mg/kg doses of ketamine were superior to the active placebo comparator, supporting its use as a novel antidepressant therapy. Interestingly, the lower doses that were tested did not separate from the placebo comparator. This is study is incredibly important because previously no dose range finding studies have been conducted with ketamine in patients with TRD. Their data suggests that clinicians who use lower doses of ketamine may not see any significant clinical response. This is typical of most drugs and is an important step toward developing the ideal dosing regimen that balances clinical benefit and risk.
If you or someone you know is depressed, please make an appointment to see if you are a good candidate for ketamine. Dr. Ashraf Hanna is located in Clearwater, FL and is one of the leading experts in IV ketamine therapy. Dr. Hanna is a licensed anesthesiologist and Director of Pain Management at the Florida Spine Institute. He has performed thousands of infusions and patients travel from all over the world to benefit from his expertise. If you want to learn more, visit his website and listen to his patient testimonies. You won’t be disappointed! What are you waiting for? Make an appointment today!
by admin | Aug 25, 2018 | Uncategorized
The National Institute of Mental Health (NIMH) issued a highlight on ketamine for treating depression.
The most commonly used antidepressants are largely variations on a theme; they increase the supply within synapses of a class of neurotransmitters believed to play a role in depression. While these drugs relieve depression for some, there is a weeks-long delay before they take effect, and some people with “treatment-resistant” depression do not respond at all.
The delay in effectiveness has suggested to scientists that the medication-induced changes in neurotransmitters are several steps away from processes more central to the root cause of depression. One possibility for a more proximal mechanism is glutamate, the primary excitatory, or activating, neurotransmitter in the brain. Preliminary studies suggested that inhibitors of glutamate could have antidepressant-like effects, and in a seminal clinical trial, the drug ketamine—which dampens glutamate signaling—lifted depression in as little as 2 hours in people with treatment-resistant depression.34
The discovery of rapidly acting antidepressants has transformed our expectations—we now look for treatments that will work in 6 hours rather than 6 weeks. But ketamine has some disadvantages; it has to be administered intravenously, the effects are transient, and it has side effects that require careful monitoring. However, results from clinical studies have confirmed the potential of the glutamate pathway as a target for the development of new antidepressants. Continuing research with ketamine has provided information on biomarkers that could be used to predict who will respond to treatment.35 Clinical studies are also testing analogs of ketamine in an effort to develop glutamate inhibitors without ketamine’s side effects that can then be used in the clinic.36 Ketamine may also have potential for treating other mental illnesses; for example, a preliminary clinical trial reported that ketamine reduced the severity of symptoms in patients with PTSD. 37 Investigation of the role of glutamate signaling in other illnesses may provide the impetus to develop novel therapies based on this pathway.

Left: Change in the 21-item Hamilton Depression Rating Scale (HDRS) following ketamine or placebo treatment.
Right: Proportion of responders showing a 50 percent improvement on the HDRS following ketamine or placebo treatment.34
Source: Carlos Zarate, M.D., Experimental Therapeutics and Pathophysiology Branch, NIMH
One of the imperatives of clinical research going forward will be to demonstrate whether the ability of a compound to interact with a specific brain target is related to some measurable change in brain or behavioral activity that, in turn, can be associated with relief of symptoms. In a study of ketamine’s effects in patients in the depressive phase of bipolar disorder, ketamine restored pleasure-seeking behavior independent from and ahead of its other antidepressant effects. Within 40 minutes after a single infusion of ketamine, treatment-resistant depressed bipolar disorder patients experienced a reversal of a key symptom—loss of interest in pleasurable activities—which lasted up to 14 days.38 Brain scans traced the agent’s action to boosted activity in areas at the front and deep in the right hemisphere of the brain. This approach is consistent with the NIMH’s RDoC project, which calls for the study of functions—such as the ability to seek out and experience rewards—and their related brain systems that may identify subgroups of patients with common underlying dysfunctions that cut across traditional diagnostic categories.
The ketamine story shows that in some instances, a strong and repeatable clinical outcome stemming from a hypothesis about a specific molecular target (e.g., a glutamate receptor) can open up new arenas for basic research to explain the mechanisms of treatment response; basic studies can, in turn, provide data leading to improved treatments directed at that mechanism. A continuing focus on specific mechanisms will not only provide information on the potential of test compounds as depression medications, but will also help us understand which targets in the brain are worth aiming at in the quest for new therapies.
If you or someone you know is depressed, please make an appointment to see if you are a good candidate for ketamine. Dr. Ashraf Hanna is located in Clearwater, FL and is one of the leading experts in IV ketamine therapy. Dr. Hanna is a licensed anesthesiologist and Director of Pain Management at the Florida Spine Institute. He has performed thousands of infusions and patients travel from all over the world to benefit from his expertise. If you want to learn more, visit his website and listen to his patient testimonies. You won’t be disappointed! What are you waiting for? Make an appointment today!
by admin | Aug 14, 2018 | Uncategorized

Researchers from the University of Minnesota and The Mayo Clinic found that ketamine caused an average decrease of 42% on the Children’s Depression Rating Scale(CDRS)—the most widely used rating scale in research trials for assessing the severity of depression and change in depressive symptoms among adolescents. The study recruited adolescents, 12-18 years of age, with treatment-resistant depression (TRD; failure to respond to two previous antidepressant trials). The teens were administered intravenous ketamine (0.5 mg/kg) by infusion six times over two weeks.
The study reported that the average decrease in CDRS-R was 42.5% (p = 0.0004). Five (38%) adolescents met criteria for clinical response (defined as >50% reduction in CDRS-R). Three responders showed sustained remission at 6-week follow-up; relapse occurred within 2 weeks for the other two responders. The ketamine infusions were generally well tolerated; dissociative symptoms and hemodynamic symptoms were transient. Interestingly, higher dose was a significant predictor of treatment response.
“Adolescence is a key time period for emergence of depression and represents an opportune and critical developmental window for intervention to prevent negative outcomes,” the authors wrote in the study.
“Unfortunately, about 40% of adolescents do not respond to their first intervention and only half of non-responders respond to the second treatment,” they said. “Because standard interventions require prolonged periods (e.g., weeks to months) to assess efficacy, serial treatment failures allow illness progression, which in turn worsens the outcome. Hence, novel treatment strategies to address treatment-resistant depression in adolescents are urgently needed.”
The authors concluded that their results demonstrate the potential role for ketamine in treating adolescents with TRD. Additionally, evidence suggested a dose–response relationship; future studies are needed to optimize dose.
If you or someone you know is depressed, please make an appointment to see if you are a good candidate for ketamine. Dr. Ashraf Hanna is located in Clearwater, FL and is one of the leading experts in IV ketamine therapy. Dr. Hanna is a licensed anesthesiologist and Director of Pain Management at the Florida Spine Institute. He has performed thousands of infusions and patients travel from all over the world to benefit from his expertise. If you want to learn more, visit his website and listen to his patient testimonies. You won’t be disappointed! What are you waiting for? Make an appointment today!
by admin | Aug 8, 2018 | Uncategorized

Researchers at Yale published a new study titled “Acute and Longer-Term Outcomes Using Ketamine as a Clinical Treatment at the Yale Psychiatric Hospital” in Clinical Psychiatry. In late 2014, Yale began providing ketamine as an off-label therapy on a case-by-case basis for patients who could not participate in research protocols. The authors observed 54 patients that received IV ketamine infusion for the treatment of severe and treatment-resistant mood disorders such as depression.
“Ketamine is being used as an off-label treatment for depression by an increasing number of providers, yet there is very little long-term data on patients who have received ketamine for more than just a few weeks,” Samuel T. Wilkinson, MD,from the department of psychiatry, Yale School of Medicine and Yale Psychiatric Hospital, told Healio Psychiatry.
The Yale researchers studied the acute and longer-term outcomes in this patient population. Importantly, a subset of patients (n=14) received ketamine on a long-term basis, ranging from 12 to 45 total treatments, over a course of 14 to 126 weeks. The researchers found no evidence of cognitive decline, increased proclivity to delusions, or emergence of symptoms consistent with cystitis in this subset of long-term ketamine patients. They also reported that the infusions were generally well-tolerated.
Although this study population was relatively small, limiting the conclusions that can be drawn, this is still an important first step in establishing the long-term safety of ketamine for the treatment of a myriad of diseases that it’s being used to treat.
If you or someone you know is depressed, please make an appointment to see if you are a good candidate for ketamine. Dr. Ashraf Hanna is located in Clearwater, FL and is one of the leading experts in IV ketamine therapy. Dr. Hanna is a licensed anesthesiologist and Director of Pain Management at the Florida Spine Institute. He has performed thousands of infusions and patients travel from all over the world to benefit from his expertise. If you want to learn more, visit his website and listen to his patient testimonies. You won’t be disappointed! What are you waiting for? Make an appointment today!
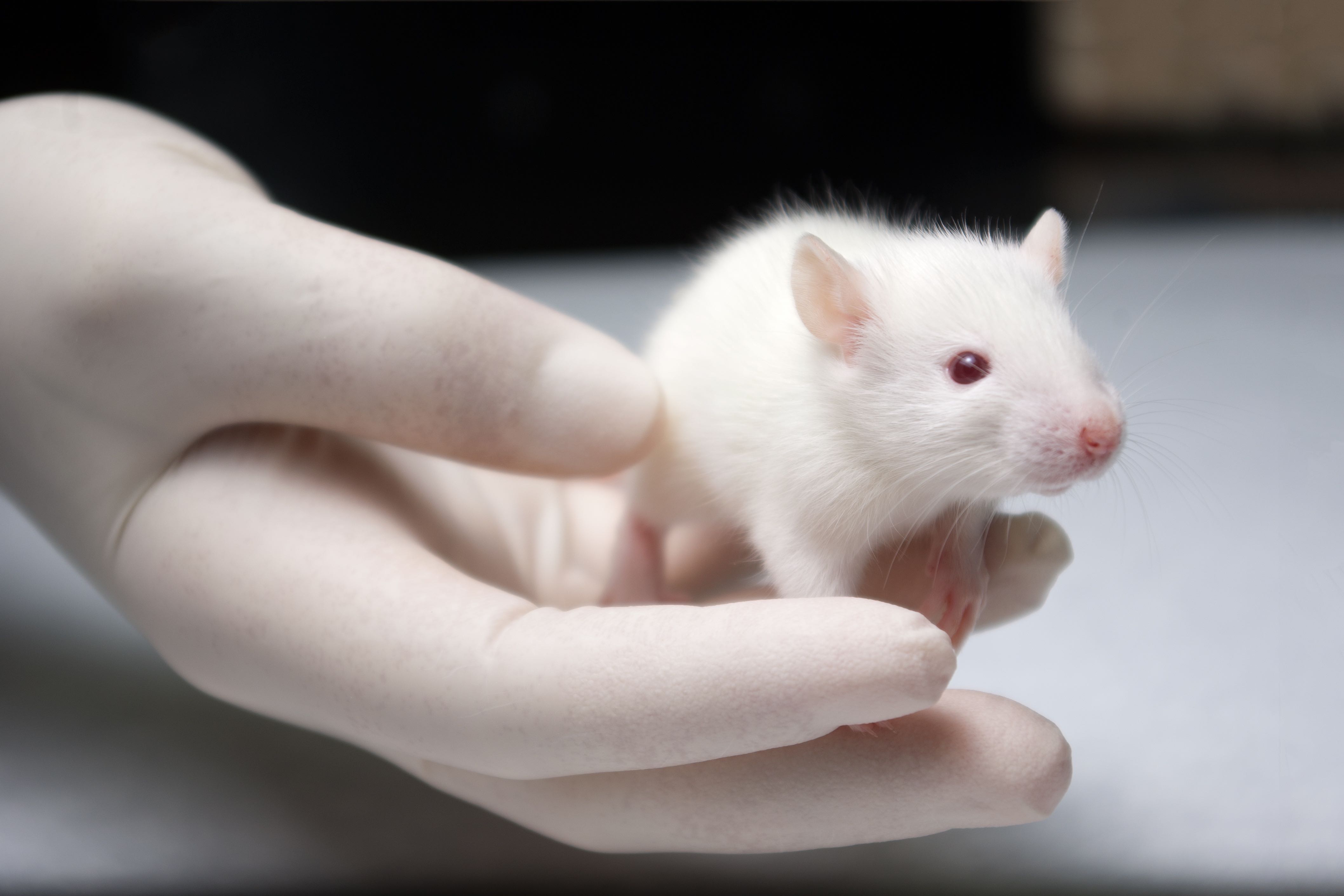
by admin | Dec 7, 2015 | Uncategorized
People born with a rare genetic mutation are unable to feel pain, but previous attempts to recreate this effect with drugs have had surprisingly little success. Using mice modified to carry the same mutation, UCL researchers funded by the MRC and Wellcome Trust have now discovered the recipe for painlessness.
‘Channels’ that allow messages to pass along nerve cell membranes are vital for electrical signalling in the nervous system. In 2006, it was shown that sodium channel Nav1.7 is particularly important for signalling in pain pathways and people born with non-functioning Nav1.7 do not feel pain. Drugs that block Nav1.7 have since been developed but they had disappointingly weak effects.
The new study, published in Nature Communications, reveals that mice and people who lack Nav1.7 also produce higher than normal levels of natural opioid peptides.
To examine if opioids were important for painlessness, the researchers gave naloxone, an opioid blocker, to mice lacking Nav1.7 and found that they became able to feel pain. They then gave naloxone to a 39-year-old woman with the rare mutation and she felt pain for the first time in her life.
“After a decade of rather disappointing drug trials, we now have confirmation that Nav1.7 really is a key element in human pain,” says senior author Professor John Wood (UCL Medicine). “The secret ingredient turned out to be good old-fashioned opioid peptides, and we have now filed a patent for combining low dose opioids with Nav1.7 blockers. This should replicate the painlessness experienced by people with rare mutations, and we have already successfully tested this approach in unmodified mice.”
Broad-spectrum sodium channel blockers are used as local anaesthetics, but they are not suitable for long-term pain management as they cause complete numbness and can have serious side-effects over time. By contrast, people born without working Nav1.7 still feel non-painful touch normally and the only known side-effect is the inability to smell.
Opioid painkillers such as morphine are highly effective at reducing pain, but long-term use can lead to dependence and tolerance. As the body becomes used to the drug it becomes less effective so higher doses are needed for the same effect, side effects become more severe, and eventually it stops working altogether.
“Used in combination with Nav1.7 blockers, the dose of opioid needed to prevent pain is very low,” explains Professor Wood. “People with non-functioning Nav1.7 produce low levels of opioids throughout their lives without developing tolerance or experiencing unpleasant side-effects. We hope to see our approach tested in human trials by 2017 and we can then start looking into drug combinations to help the millions of chronic pain patients around the world.”
The findings were made possible by the use of ‘transgenic’ mice, meaning they were modified to carry genetic material from another organism — in this case, the mutation that prevents humans from feeling pain. Precise physiological experiments showed that the nervous systems of the transgenic mice contained around twice the levels of naturally-produced opioids as unmodified mice from the same litter.
“Our results reaffirm the clinical relevance of transgenic mouse models for human diseases,” says Professor Wood. “Studying the mice showed us what was going on in the nervous system that led to painlessness and our findings were directly translatable to humans, as confirmed by the painless patient. Without the work in transgenic mice, none of this would have been possible and we still wouldn’t know how to replicate the effects to help people suffering from chronic pain.”
Story Source:
University College London. “Genetically modified mice reveal the secret to a painless life: Researchers have discovered the pharmaceutical recipe for painlessness.” ScienceDaily. ScienceDaily, 4 December 2015. <www.sciencedaily.com/releases/2015/12/151204090034.htm>
Journal Reference:
- Michael S. Minett, Vanessa Pereira, Shafaq Sikandar, Ayako Matsuyama, Stéphane Lolignier, Alexandros H. Kanellopoulos, Flavia Mancini, Gian D. Iannetti, Yury D. Bogdanov, Sonia Santana-Varela, Queensta Millet, Giorgios Baskozos, Raymond MacAllister, James J. Cox, Jing Zhao, John N. Wood. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nature Communications, 2015; 6: 8967 DOI: 10.1038/ncomms9967





